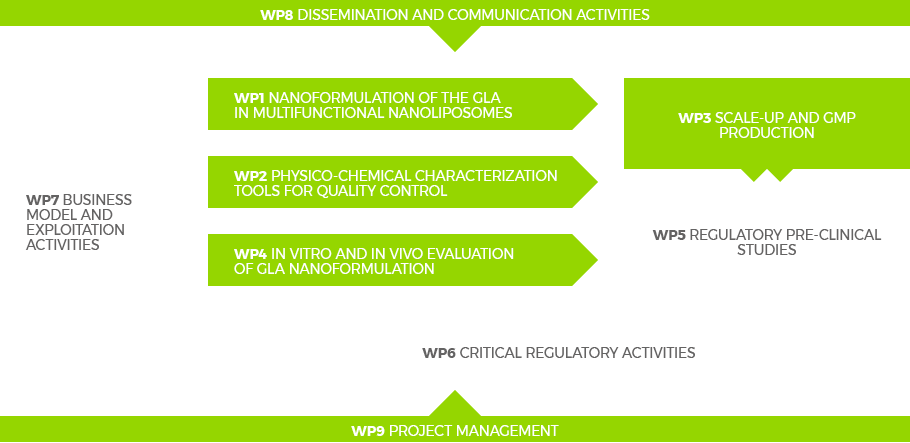
WP6
CRITICAL REGULATORY ACTIVITIES
Medicinal products regulation is a critical issue for the development of innovative pharmaceutical production processes and products. In this sense, Smart-4-Fabry will follow the regulatory requirements in the development of GLA-nanoformulation,
including formulation, manufacturing and nonclinical tests. The regulatory requirements will be implemented according to the expert partner (DDR) by providing appropriate inputs to any of the WP, and by ensuring frequent interaction with the relevant European regulators in the form of scientific advice procedures.
WP7
BUSINESS DEVELOPMENT AND EXPLOITATION
A detailed business plan will be implemented, together with an update of the already developed market study, including:
identification of key exploitable results, IPR management, market assessment (size, volume, distribution), constitution of an exploitation team, detailed financial projections, taking into account remaining costs for clinical development, detailed cost evaluation for the production process and the obtained products, marketing costs and foreseen revenue, commercialization roadmap and risk assessment. This business plan will be supported by a detailed Exploitation Plan in order to catch all the exploitable items to be transferred to markets or to be serve as a basis for future R&D efforts. Exploitation will be understood in a wide manner at different levels, as exploitation interest will differ between industrial and academic partners, but in a full complementary way.